12 COVID19 vaccines across the globe crossing Phase III – Greater hope for widespread access?
“12 COVID19 vaccines crossing Phase III clinical milestones, interim readouts from 4 candidates  with trial sites spanning US, UK, Brazil and Russia, global breadth of clinical pipeline, technology diversity – great promise for access to COVID19 vaccine for priority groups by March 2021 and for populations at large by Fall 2021. Looming questions remain on logistical challenges, manufacturing scale-up roadmap for equitable global access and long term safety. Yet, November proves to be harbinger of hope in the COVID19 vaccine journey”
with trial sites spanning US, UK, Brazil and Russia, global breadth of clinical pipeline, technology diversity – great promise for access to COVID19 vaccine for priority groups by March 2021 and for populations at large by Fall 2021. Looming questions remain on logistical challenges, manufacturing scale-up roadmap for equitable global access and long term safety. Yet, November proves to be harbinger of hope in the COVID19 vaccine journey”
In less than a year from being declared a Global Health Emergency by the World Health Organization (WHO), global scientific momentum has led to a robust pipeline of 48 COVID19 vaccines in various stages of clinical development and 164 preclinical candidates. This sets a new benchmark in the pace of vaccine development; and reflects intensity of global efforts, corporate commitment, policy and philanthropic encouragement and power of technology platforms such as mRNA and DNA vaccines.
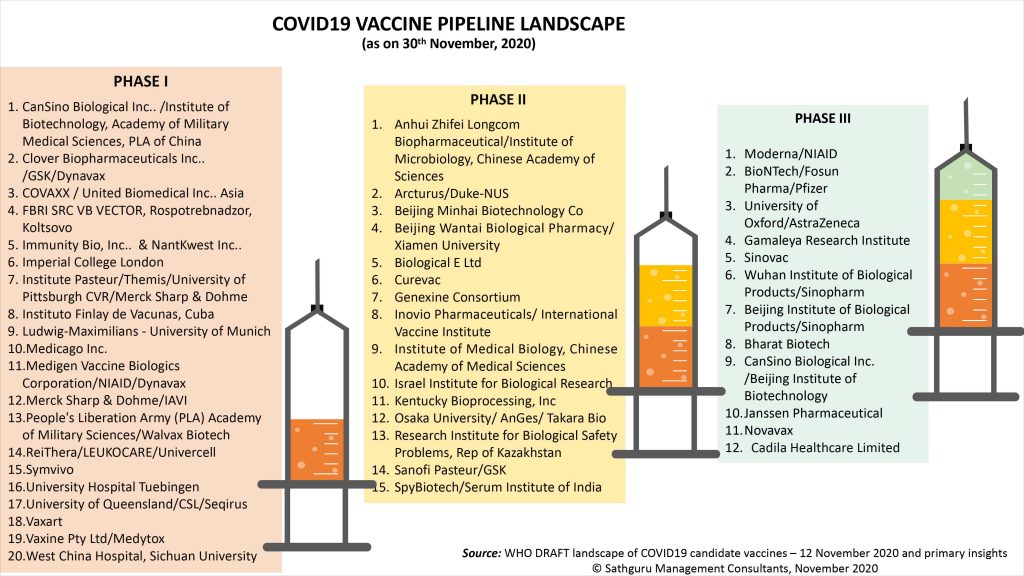
The image below represents various vaccines across stages of clinical development:
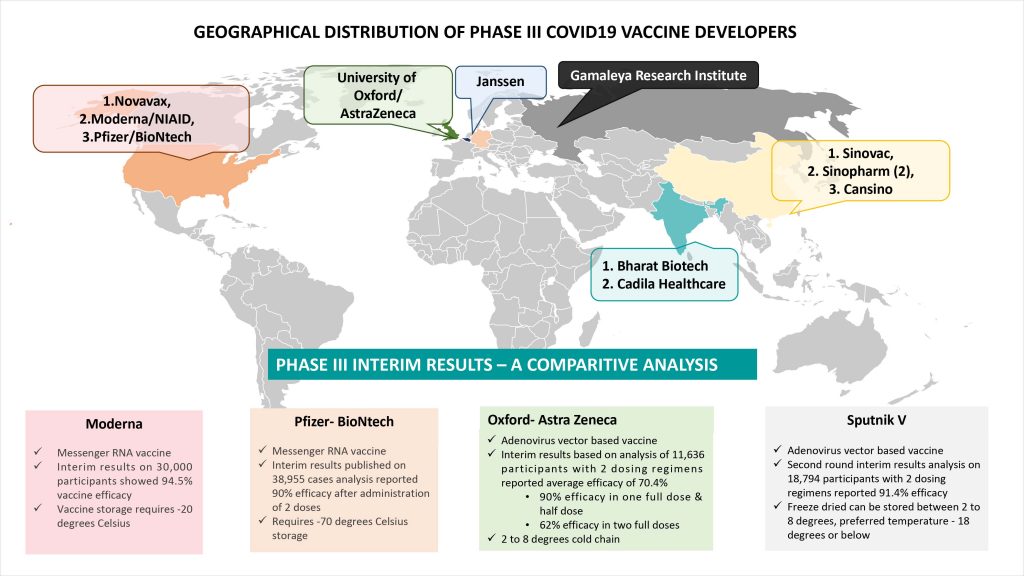
Geographic diversity of Phase III pipeline of COVID19 vaccines critical for access at unprecedented scale
It is highly encouraging to note that as of November end, 12 COVID19 vaccines are pushing through Phase III clinical trials (Source – WHO COVID Vaccine landscape dated 12 November). November’s progress implies high promise of COVID19 vaccine access for priority groups (healthcare workers, senior citizens etc.) across geographic regions by March 2021 and ramped up access for global populations by Fall 2021.
In addition to breadth of pipeline at this final stage of development, the geographic spread of vaccine developers involved in this effort is an important harbinger of hope. The pipeline of twelve candidates includes three from big pharma (AstraZeneca in partnership with UK’s University, Pfizer in partnership with German biotech BioNTech and Janssen, two Indian companies (Bharat Biotech in collaboration with India’s ICMR and Cadila Healthcare with its DNA vaccine), two American ventures that have shot to spotlight with the COVID19 pandemic (Moderna and Novavax) and four Chinese vaccines. Several of these Phase III COVID19 vaccines are being validated in South America and have forged production partnerships across North America, Europe and Asia. With no global region spared from the onslaught of the COVID19 virus, this geographic breadth of the Phase III pipeline is critical for equitable global access.
Technology breadth in the global Phase III pipeline of COVID19 has been important to defray development risk while supporting acceleration. Global forerunners, Moderna and Pfizer / BioNtech demonstrate the potential of mRNA platform for vaccines. A technology platform where oncology investments have dwarfed pursuit of vaccines, COVID19 is a resounding reminder that it is here to stay. The same is true of DNA vaccines – again with several advanced candidates including Inovio, Osaka University / Takara Bio and Genexine Consortium. Long standing platform investments in mRNA and DNA technologies have finally borne fruit and allowed opportunistic application in an accelerated manner. Case in point is India’s Cadila Healthcare, a company that gained platform strength in viral vaccines through the acquisition of Etna Biotech (Italy based vaccine R&D arm of Crucell) in 2008 and is now leaping on the Phase III wagon in India. At the end other end, established platforms used by Novavax, Oxford University, Bharat Biotech/ICMR and Sinovax provided much needed technology de-risking with proven safety profile of their development approach. This diversity of next generation and established technology approaches provides Governments and patients the possibility of contextually judicious use. This is especially relevant while they make choices under public health and economic pressures but in the absence of long term safety data. The diverse technology platforms also implies varying operating considerations such as cold chain requirement, production scale-up etc.
Phase III interim result readouts infuse confidence despite looming questions
November marked a major milestone in clinical development of COVID19 vaccines. Four global candidates – Pfizer / BioNTech, Moderna, Astra Zeneca / Oxford University and Gamaleya Research Institute have announced interim results from the ongoing Phase III trials. More details on the interim results readouts of the COVID-19 vaccine are available in our Pharm Forward website here.
While the forerunners’ promising results with trial data emerging from US, UK, Brazil and Russia provide assuring solace for both public health and economic health, they are currently shrouded in several looming questions and controversies. Amongst the unanswered parts of the maze, the three that dominate today are: 1. Practical considerations around logistics and delivery, 2. Pathway to manufacturing scale-up 3. Mixed results in the AstraZeneca-Oxford trial and implications thereof.
Logistical questions loom large on the Pfizer/BioNTech and Moderna mRNA vaccines that need to be moved at -70 degrees Celsius and -20 degrees Celsius respectively. Limited scale-up manufacturing partnership have been forged by both till date and the world waits for greater visibility on how these forerunners can serve a greater share of the global demand. AstraZeneca stands out for its commitment to worldwide access at a reasonable price and astute forging of the manufacturing partnership with India’s Serum Institute, the largest manufacturer of vaccines globally. However, its Phase III data is clouded with questions around widely different efficacy endpoint from two different arms of the trial, one with 90% efficacy and the other with 62% efficacy. Compounding the data analysis and interpretation is the complexity of varying dosages erroneously being used in one, the 90% efficacy arm having a half (first) dose. Finally, given the limited duration of data tracking, it will be a while before we understand the duration of immunity provided by each of these vaccines, need for subsequent booster doses and long term safety considerations.
Unprecedented global demand, beginning of the vaccination journey and need for continued global collaboration:
Volume demand for the COVID19 is unprecedented given the global onslaught of the virus. With two doses being the most common dosing schedule being pursued globally, aggregate global demand is north of 10 billion doses. Adding to this volume challenge is the dearth in large scale manufacturing capacity with these vaccines required to be manufactured at BSL II level of biosafety compliance.
While the world could start delivering first doses to COVID19 vaccine to certain high risk groups as early as December 2020, we have a long path to traverse in this journey of immunizing large cohorts of global population in the context of the pandemic. While we intently focus on accelerating and ramping up commercial production of vaccines that are closer to the finish line, we also derive great hope from possibility offered by the second wave of vaccines such as the COVAXX’s peptide vaccine that can be relatively easily scaled up and has demonstrated superlative non-clinical data so far. Global breadth of vaccines again is a great source of encouragement as we combat the globally dominant COVID19 crisis.
The robust clinical development pipeline which will translate to widespread vaccine availability only when they can be commercially delivered at scale right after product licensure. With majority of the vaccine developers not having an in-house production capability and most countries not capable of handling vaccines that require ultra-low temperatures, meaningful partnerships are the way forward to ensure equitable and timely access to vaccines. The last word: in partnerships we trust.
 Grow Beyond
Grow Beyond