
AN UPDATE ON COVID-19 MANAGEMENT – THERAPEUTICS AND VACCINES
While some regions claim to have bent the curve, the war against the COVID-19 pandemic is not even half done. As of 17th April, the world has reported more than 2.1 million cases and 145k deaths. We are still racing to develop validated therapeutics to treat critically ill patients and a vaccine to provide a more sustainable solution. Following up on our previous newsletter, we have summarized for you validated facts and details on global developments around preventive and curative therapies for COVID – 19.
With optimism that science led solutions will turn the tide around.
Team Sathguru
In our previous newsletter we discussed how anti-viral drugs utilized for treating other conditions are being tested as a potential cure for COVID-19. Since, these drugs have already undergone numerous trials for assessing their efficacy, bioavailability and pharmacodynamics, launching them for a new condition will be entail lower costs with shorter regulatory pathways. The candidates currently being tested are anti-malarial agents and protein inhibitors that have been used for the treatment of other viral diseases such as Influenza, SARS, MERS, and HIV.
Three candidates, from Fujifilm Toyama Chemical, Gilead Science and NIH respectively, are currently conducting Phase III trials on their antiviral drugs. Fujifilm’s Avigan (generic name: favipiravir) is a broad spectrum RNA replicase inhibitor, used to treat Influenza. Upon request by the Chinese and Japanese Health Ministries, trial is carried out to assess its efficacy and safety (NCT04336904). Gilead’s Remdesivir is a nucleotide analogue indicated for the management of Ebola. Since its first activity against coronavirus was recorded in 2017, this drug is being tested as a potential treatment for COVID-19 (NCT04321616). Similarly, NIH has collaborated with National Heart, Lung, and Blood Institute (NHLBI) to conduct Phase III trial of Hydroxychloroquine, an antimicrobial agent with immunomodulatory and antiviral propertieswhich has been used for the management of malaria, autoimmune conditions like rheumatoid arthritis and lupus erythematosus (NCT04332991). Recently, USFDA has issued an emergency use authorization for the use of hydroxychloroquine to treat adults and adolescents who weigh 50 kg or more and are hospitalized with COVID-19 for whom a clinical trial is not available, or participation is not feasible.
Other antiviral drug acing in the league is the adenosine analogue Galidesivir, developed by BioCryst Pharmaceuticals. While animal studies, have shown that the drug was effective in treating conditions including Ebola, Marburg, Yellow Fever and Zika viruses; Phase I trials are being planned (NCT03891420).
Global collaborative efforts to accelerate validation and informed clinical use:
In the midst of global crisis, there has been a lot of thrust on coordinated global trials and pooled national efforts to gain the much needed acceleration advantage. The most significant one is the WHO solidarity trial that aims to assess the effectiveness of Remdesivir; Lopinavir/Ritonavir; Lopinavir/Ritonavir with Interferon beta-1a; and Chloroquine or Hydroxychloroquine in the treatment of COVID-19. On similar lines, NHS, UK has rolled out the Recovery trial in collaboration with the University of Oxford to test the efficacy of Lopinavir-Ritonavir; low dose dexamethasone; hydroxychloroquine and azithromycin. Clinical networks and coordinated trials hold highest promise to accelerate data generation from patients being treated world wide. With expanding participation from countries, there is great promise of clinicians across the world potentially being equipped with the arsenal of validated drugs and clinical outcomes improving.
Plasma Replacement Therapy or Convalescent Plasma Therapy is an investigative clinical initiative driven by healthcare delivery institutions globally to use plasma from recovered patients as an in-hospital therapy for critical patients. When a person gets a viral infection, that person’s body produces proteins called antibodies to fight off the virus. These antibodies present in the blood plasma continue to circulate even after the person has recovered and could be used to treat patients who are currently infected. The approach has been advocated by clinicians across borders and is being used as an investigative therapy in several countries. The US consortium led by the Michigan State University has multiple academic centers participating and the USFDA has requested recovered patients to donate plasma. France and Germany also have trails ongoing. ICMR in India has also initiated a trial in India and rolled out a letter of intent urging institutions to collaborate in the study. Similarly, the Dubai Health Authority (DHA) has also recently declared initiation of a protocol for convalescent plasma therapy. While we still don’t have data from the clinical trials validating the clinical benefit across population groups, we are enthused by near term promise of this approach.
Companies have also been active to join the global effort to explore potential of polyclonal hyper immune globulin (H-IG) against SARS-CoV-2 that is recovered from the plasma of recovered patients. Involvement of industry will offer higher scalability, availability and standardization of these products, thereby improving access for hospitals that have limited in-house capacity for delivering such plasma based therapies. Some of the significant yet early stage players include Takeda (in collaboration with Biotest, Bio Products Laboratory, Octapharma and LFB), Emergent BioSolutions (with BARDA), Grifols (with BARDA) and Kamada. As an alternate in-hospital approach, USFDA has also granted an emergency authorization to a blood purification system to Terumo BCT Inc. and Marker Therapeutics AG for their Spectra Optia Apheresis System and Depuro D2000 Adsorption Cartridge devices. The device is used to treat COVID-19 patients with confirmed or imminent respiratory failure and will expedite availability of services in ICU. The device works by reducing the amount of cytokines and other inflammatory mediators, thereby reducing inflammation that leads to organ failure and death.
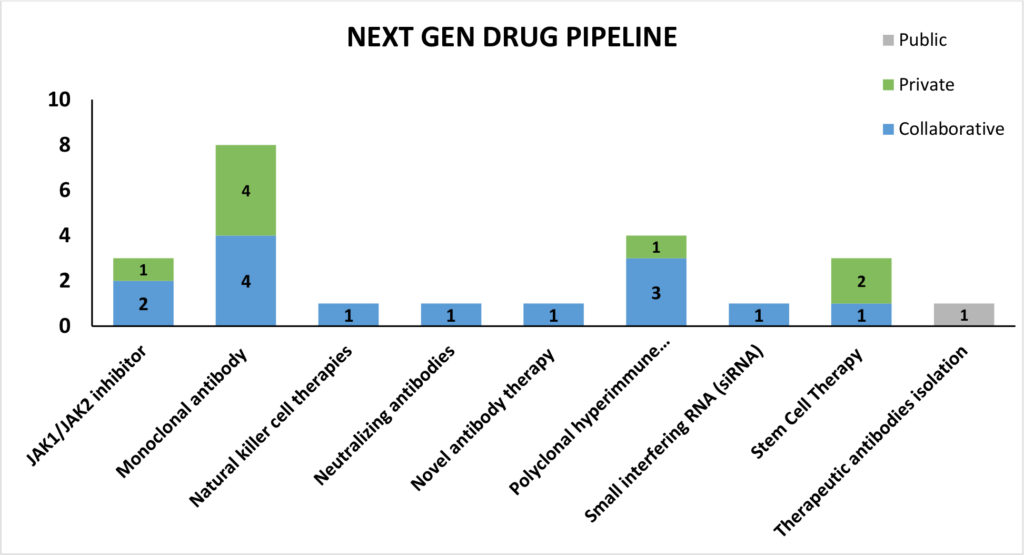
In our March newsletter we discussed the growing focus on next-generation therapies with several regenerative medicine companies exploring the potential of stem cell therapy in preventing and treating fatal ARDS associated with late-stage coronavirus disease. We have summarized next generation therapeutic technologies currently under development:
Stem cell therapies
Australia based Mesoblast received USFDA’s clearance to treat COVID-19 patients using its allogeneic anti-inflammatory mesenchymal stem cell product (MSC) remestemcel-L. The USFDA has given the green signal to use the drug under both expanded access compassionate use and in a planned randomized controlled trial. The MSC candidate has previously been successful in phase-III trials for steroid-refractory acute graft versus host disease (aGVHD) in children, a potentially fatal inflammatory condition due to a similar cytokine storm process as is seen in COVID-19 ARDS.
USFDA authorized another candidate, MultiStem by Athersys, to initiate pivotal phase II/III clinical trial in patients with COVID-19 induced ARDS. MultiStem is developed from a special class of stem cells called Multipotent Adult Progenitor Cells, or MAPC® that are obtained from healthy adult bone marrow. In May 2019, the candidate got USFDA Fast Track Designation on successful phase I/II study evaluating its use in ARDS.
Amidst the other next generation therapies that are currently in early investigational stage, some of the candidates follow different mechanism of action. For instance, one candidate is targeted at eliminating the infection by limiting SARS-CoV-2 replication. Celularity’s CYNK-001, a cryopreserved allogenic, off-the-shelf Natural Killer (NK) cell therapy that is developed from placental hematopoietic stem cells received USFDA’s clearance to conduct phase I/II clinical trial including up to 86 patients with COVID-19. NK cells are associated with a range of biological functions during an infection like the expression and activation of receptors that bind to stress ligands active during an infection and viral antigens on infected cells and limit their replication and progression of infection.
Biologics – Monoclonal Antibodies based therapies
Actemra (generic name: Tociliumab), an interleukin-6 antibody, developed by Roche has recently received USFDA approval to conduct Phase III trials. Currently, the drug has been approved by the USFDA for treatment of cytokine release syndrome (CRS). Expected to begin enrolment in April 2020, the trial will be conducted in partnership with BARDA (NCT04320615).
Another candidate, Kevzara (generic name: Sarilumab) is a monoclonal antibody that inhibits the interleukin-6 (IL-6) pathway by binding and blocking the IL-6 receptor. The trial under partnership of Sanofi and Regeneron Pharmaceuticals, is expected to begin soon, wherein Regeneron will lead the trial within US and Sanofi outside of US (NCT04315298).
Cytodyn’s IgG4 monoclonal antibody, Leronlimab, is a CCR5 antagonist that has shown dramatic immune restoration, especially in the CD8 T-lymphocyte population after 7 days of treatment. Working in partnership with Progenics Pharmaceuticals, this drug has scheduled to conduct Phase II/III trials shortly (NCT04347239).
Recently, Eli Lilly has also announced a Phase II trial on LY3127804, an investigational selective monoclonal antibody against Angiopoietin 2 (Ang2), to study its effectiveness in treating pneumonia patients hospitalized with COVID-19 who are at a higher risk of progressing to acute respiratory distress syndrome (ARDS). Ang2 is known to be elevated in ARDS patients and Lilly will test whether inhibiting the effects of Ang2 with a monoclonal antibody can reduce the progression to ARDS or the need for mechanical ventilation in COVID-19 patients.
InflaRx in conjunction with Beijing Defengrei Biotehnology Co. Ltd., has reported success in Phase I trial of IFX-1, a monoclonal antibody targeting the complement activation product C5a. Acting as an immune-modulator, this drug is proven safe and well tolerated in a double-blind placebo-controlled dose escalation Phase I study in healthy human volunteers. The company has recently received approval to conduct Phase II/III trial (NCT04333420).
| Developer | Type of candidate | Current Status |
| Vir Biotechnology and GSK | Monoclonal antibody | Preclinical stage |
| Celltrion and Korea Centres for Disease Control and Prevention | Monoclonal antibody | Preclinical stage |
| Junshi Bioscience and IMCAS | Neutralizing antibodies | Preclinical stage |
| Amgen And Adaptive Biotechnologies | Novel antibody therapy | Early stage |
| Anti-COVID consortium: National Institute of Immunology and Gennova Biopharmaceutical Limited | Therapeutic antibodies isolation | Early stage |
| Biogen and Vir Therapeutics | Monoclonal antibody | Early stage |
Immunomodulating JAK Inhibitors
Amongst other candidates, Pfizer’s JAK inhibitor tofacitinib has been authorized to conduct Phase II trials (NCT04332042) in patients with COVID-19 related pneumonia. The drug inhibits essential cytokine signalling involved in immune-mediated inflammatory response.
Ruxolitinib, is a janus-associated kinase inhibitor indicated to treat bone marrow cancer, is currently awaiting USFDA approval to undergo Phase III trials to manage COVID-19 cases. The originator company, Incyte is working in collaboration with Novartis for conducting the study, outside US (NCT04348071).
Another industry expert, Eli Lilly is working in collaboration with National Institute of Allergy and Infectious Diseases to study Baricitinib, a JAK1/JAK2 inhibitor, as a potential treatment for hospitalized patients diagnosed with COVID-19. The preclinical trials are expected in the next quarter.
Some of the other noteworthy advances have been summarized below:
| Developer | Type of candidate | Current Status |
| Apeiron Biologic | Recombinant Human Angiotensin-converting Enzyme 2 (rhACE2) which has the potential to block the entry of virus into the cell. | Phase II approval received (NCT04335136) |
| Emory University and Ridgeback Biotherapeutics | Broad spectrum antimicrobial that has proven effective activity against influenza, SARS, MERS, chikungunya and equine encephalitis. Acting as a potent ribonucleotide analogue, it prevents RNA replication. | IND application |
| Neuroclear Technologies Inc. | Broad spectrum antiviral drug that inhibits Inosine monophosphate dehydrogenase, thus suppressing SARS-CoV-2 replications. | IND application |
| Enanta Pharmaceuticals | Utilizing their discovery of protease inhibiting antivirals for HCV, and HBV treatment in the management of COVID-19. | Early stage |
| Innovation Pharmaceuticals | The company will test its defensin-mimetic drug candidate, Brilacidin for potential anti-viral properties. | Early stage |
| Resverlogix Corporation | Currently testing on Apabetalone, a Bromodomain and extraterminal domain protein inhibitor that prevents expression of the pathogen. | Early stage |
| Redhill Biopharma Ltd. and Apogee Biotechnology Corp. | A sphingosine kinase (SK) 2 and dihydroceramide desaturase inhibitor that reduces the C-reactive protein levels and limits inflammatory responses. | Early stage |
| Sorrento Therapeutics Inc. and University of Texas | ACE modulators that neutralize and block the pathogen thus preventing its expression. | Early stage |
| Nanoviricides | The candidate can develop ligands that bind to the virus in the same way as a cognate receptor and attack at various. | Early stage |
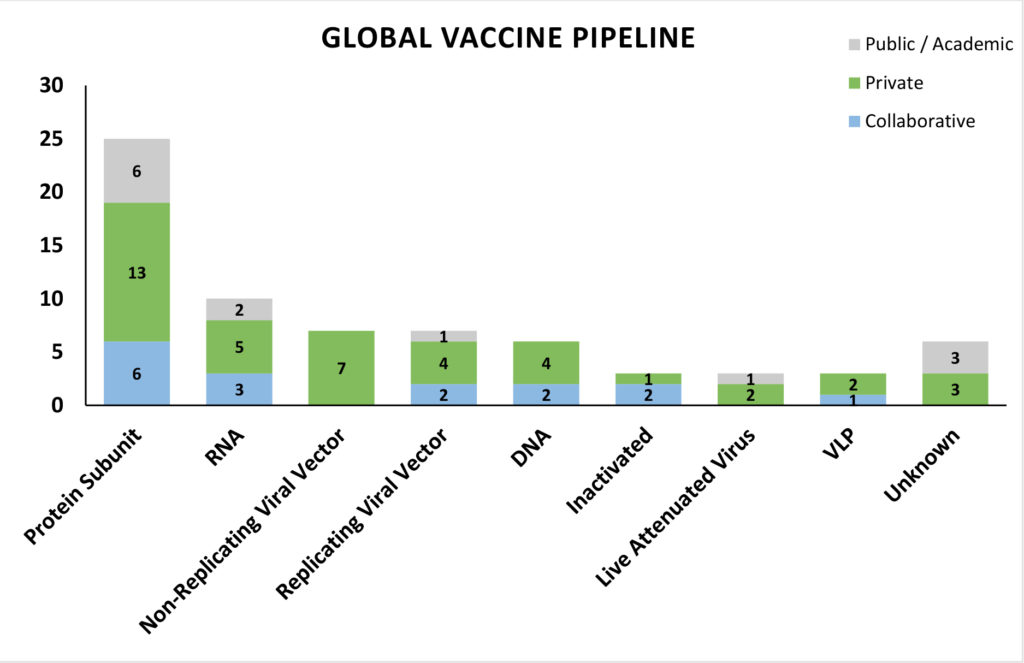
In our previous newsletter we discussed the status of response from the biopharma fraternity, and indicated details of two vaccine candidates that had already entered into phase I trials – Moderna’s mRNA vaccine (NCT04283461) and CanSino Biologics’ recombinant adenovirus vector based vaccine (NCT04313127). This week we bring more updates on their status and additional details in the landscape of vaccine development for COVID-19.
A brief analysis of the vaccine platforms being utilized in the pipeline candidates shows that most of the preclinical candidates are protein subunit vaccines targeting the S (spike) protein of SARS-CoV-2. The safety profile, stability, ease of production of subunit vaccines with safer, advanced host systems and precise immune targeting could be supporting the heightened interest from the research fraternity. Nucleic acid vaccines occupy the next spot with the highest number of candidates in development, and two mRNA vaccines and one DNA vaccine candidate having entered phase I and II trials. mRNA vaccines are an exceptionally safe and versatile vaccine platform making them as one of the obvious platforms to be used in an outbreak response to produce vaccines in a short-time frame, although, there is no licensed mRNA vaccine to date. Non-replicating Adenovirus vector based vaccines also have a number of candidates, due to their safety profile and easy of scalability. With private companies contributing to a greater share of the pie of global developers, it is encouraging to note that keenness of the private sector to contribute to global efforts around the pandemic response and commit capital in an accelerated manner. Companies with pre-existing viral vector and nucleic acid platforms enjoy incumbent scientific advantage and have been able to use those to develop vaccines targeting the immunogenic S protein of SARS-CoV-2.
CanSino Biologics candidate is currently the most advanced and has leapt forward in development and is announced the phase II clinical trial plan for its Ad5-nCoV vaccine based on satisfactory preliminary results from the phase I trial. According to the phase II protocol posted on the clinical trial registry (NCT04341389), the study site will be Wuhan with 500 healthy participants. The randomized double-blind study will examine three primary endpoints: adverse reactions within the first 14 days of vaccination, serum levels of anti-SARS-CoV-2 neutralizing antibody and antibody against the coronavirus’s spike protein at day 28. The participants will further be followed over a period of six months.
Joining the league of phase I trial candidates is Inovio’s DNA vaccine (NCT04336410) encoding the virus spike protein. The vaccine will be administered via electroporation using Inovio’s proprietary hand-held device Cellectra®. The vaccine is all set to begin first-in-human trials at two sites: Philadelphia and Kansas City with up to 40 healthy volunteers. Each participant will receive two doses of INO-4800 four weeks apart, and the initial immune responses and safety data from the study are expected by late summer. Inovio has previous experience in a vaccine against Middle East Respiratory Syndrome (MERS), which was well-tolerated and produced potent antibody response in 95% of the subjects.
Two vaccine candidates developed by the Shenzhen Geno-Immune Medical Institute have begun the phase I trial: LV-SMENP (NCT04276896), a lentivirus expression system that expresses synthetic viral minigenes and immune modulatory genes to modify the dendritic cells and activate T cells; Pathogen-specific aAPC (artificial antigen presenting cells)( NCT04299724) that are modified using a lentivirus expression system expressing synthetic viral minigenes based on selected viral proteins.
University of Oxford has also initiated enrolment for the phase I/II trial (NCT04324606) of its ChAdOx1 nCoV-19 vaccine, based on a platform previously used to develop vaccine against MERS and has underwent phase I trials at Oxford. The recombinant adenovirus based vaccine is replication incompetent and has been genetically engineered to express SARS-CoV-2 spike protein S. The University has entered into a contract with an Italian CDMO Advent for the production of the vaccine for clinical studies.
Pressing on the need for a collaborative approach to speed-up vaccine development, WHO has laid down a Research & Development Blueprint for the development of diagnostics, vaccines and therapeutics against COVID-19. As a part of that, WHO has recently published the Target Product Profile (TPP) for COVID-19 vaccines, developed through a consultation process with key stakeholders in human and animal health, scientific, funding and manufacturing sectors. The TPP aims to guide researchers and manufacturers to develop vaccines for reactive use in outbreak settings and/or confer long-term immunity to high-risk populations. The TPP can be found in detail here.
The agile response from the global research and biopharma community, along with the swift regulatory support and guidance by global organizations like WHO, ICMRA gives hope that a vaccine will soon be available to confer population level immunity.
Way Forward – the need for simultaneous investment in multiple candidates, capital pooling and accelerated investment in manufacturing facilities
Vaccines are going to be critical to generate immunity in global population and arguably, is the only sustainable solution for the world to ease out of this crisis. The agile response from the global research and biopharma community, along with the swift regulatory support and guidance by global organizations like WHO, ICMRA has resulted gives optimism that the vaccine isn’t far along. However, to truly deliver the vaccine at scale to global population on war footing timelines, it is important we also quickly enhance level of focus on:
- Simultaneously pursuing multiple vaccine candidates
The robust global pipeline is supported by funding programs from multiple national Governments and multilateral / philanthropic investors. It is imperative that all our funding programs take cognizance of inherent risk in such an endeavor. While rigorous scientific review is still pertinent for judicious capital allocation to deserving research programs, each funder should ideally support multiple vaccine candidates through the capital intensive clinical validation phases. In addition to supporting multiple vaccine candidates, funders can also maximize impact by building as much efficiency as possible in the overall funding mechanism including preliminary review and follow-on review for continued funding through progressive validation phases. It is time we advance more candidates than less and ensure not an additional day than needed is lost in process inefficiencies.
- Addressing unprecedented demand – Investing in large scale manufacturing facilities at war footing
To serve as a sustainable solution to a pandemic that hasn’t spared any region of the world, we need at least 6 billion doses of the vaccine. This demand for unprecedented volume calls for urgent and renewed thinking on creating needed manufacturing capacity, especially since substantial part of the capacity could be of BSL 3 biosafety level.Collectively, even all viral vaccine manufacturing facilities combined don’t have this quantum of capacity today. The capex and capability needed to develop this manufacturing backbone for global need is very poorly understood and under-addressed in global efforts today. With the exception of the Bill and Melinda Gates Foundation, most other national and international investors haven’t pledged commitment to supporting accelerated creation of manufacturing capacity.
While clinical development risk is not yet defrayed, it is critical that facility development is commenced simultaneously alongside product development. This entails a high degree of risk on return realization on manufacturing investments in the eventuality of the candidate failing in the clinic.Manufacturing facility design is also contingent on the vaccine platform and re-deployment could be challenging. Hence, leaning purely on industry to create the manufacturing capacity needed is unlikely to deliver the ambitious result the world is seeking in 18 months. We need government and non-government investments to be directed in equal part to immediate investments in manufacturing capacity that is developed simultaneously along with the vaccine candidates.Under-investment or delayed attention to manufacturing capacity creation can undermine all our investments in vaccine development. We need to address this to today.
- Pooling capital across public, private and philanthropic sources
Finally, we need to further coordinate our efforts at capital pooling across scientific investments to ride out of this crisis. While several countries are likely to be guided by a strong desire for technology and manufacturing self-sufficiency, it is imperative we note that this is not a race against each other.If anything, it is a combined race against time. We have to more strategically pool public, private and philanthropic capital at both the national, regional and global level.A global commitment to cross-license technology, provide access to clinical networks and scientific capacity, and delivery cost effective vaccine doses for global need should be the order of the game.

Featured publication
Sathguru Foot Print
-
- CII Lifesciences Conclave Sathguru’s healthcare practice lead Pushpa Vijayaraghavan deliberates way forward for Indian Biopharma industry at the CII Lifesciences conclave
- Conference on AI, Analytics and Automation Sathguru’s healthcare practice lead Pushpa Vijaraghavan discusses AI and in-silico modeling in drug discovery and development at Express Pharma’s R&D conference
Click here to Download the PDF version
Authors:




Connect with Authors at: E-mail healthcare@sathguru.com
 Grow Beyond
Grow Beyond 

