Improving vaccines for easier delivery and addressing public health challenges
Infectious disease vaccines represent one of the most important global health investments. According to WHO, immunizations save an estimated 2.5 million lives every year from diphtheria, tetanus, pertussis (whooping cough), and measles. However, despite decades of effort, we still poke newborn kids to deliver these magic potions to prevent diseases and we are still struggling with infrastructure issues to ensure that every newborn in the world is vaccinated despite where they are located. Several efforts on improved formulations of currently available vaccines are focused on these two areas – improving ease of delivery and addressing practical problems in public health vaccination programs.
Improving ease of delivery
In the first year of its life, an infant in US gets 16 doses of vaccines and infant in India gets 13 doses of vaccines if at least the vaccines required in the national immunization schedules are given. With the exception of the oral rotavirus vaccine (in India and US) and the oral polio vaccine (in India), all the other doses entail poking the little infants. Additionally, several studies indicate the economic benefits of flu vaccination in countries with seasonal flu such as US but the fear of the needle keeps several people away from such benefits. While the needle free vaccine, is yet to be a reality, there are several efforts towards making this delivery easier. One of the initial efforts in this direction was to combine multiple vaccines into a single dose. This is an old approach; and one of the more established globally used examples is the DTP vaccine used in various combinations across the world. This trend has been further pioneered by public health procurement bodies such as GAVI with the support extended for global adoption of the pentavalent vaccine (not yet adopted in India) that combines the DTP with recombinant hepatitis B and the conjugate vaccine Haemophilu s Influenza type B.
Global efforts on delivery innovation include intradermal delivery through jet injectors, transdermal and epidermal patches and microneedle delivery among other options. The less painful intradermal vaccine and the intranasal vaccine have been introduced in the US for flu vaccines across age groups. While these offer solutions for global adoption, questions remain over the efficacy profiles produced by some of these novel delivery systems and global effort is still ongoing to improve efficacy as well as bring down cost for widespread adoption.
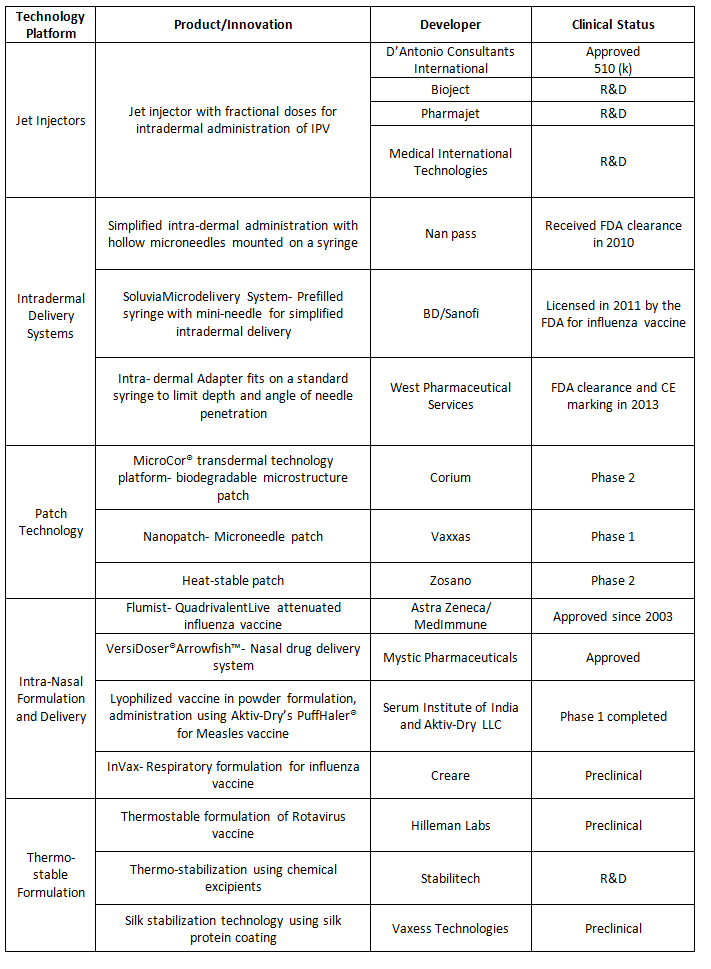
The table below highlights some key developments in the pipeline and approved products for novel delivery systems in vaccines. Finally, beyond patient convenience, novel vaccine delivery systems being developed are also focused on making vaccines safer and more effective through various approaches such as controlled release of antigens, rendering booster doses unnecessary, and improving their targeting of immune cells for increased efficacy.
Some Key Vaccine Adaptations in Development/ Approved:
Addressing public health challenges
Global public health bodies and national governments have been dealing with several practical challenges to ensure that vaccine preventable deaths can be avoided in the poorest of countries. In mid to low income countries, several capacity constraints such as lack of qualified human resource, continuous cold chain, public health infrastructure etc significantly hinder vaccination programs. While the problems itself are too large to be solved without substantial global investments, vaccine manufacturers have made several efforts to develop and incorporate novel delivery devices and formulation adaptations to address such problems.
One of the major problems in vaccination programs and a reason for high levels of vaccine wastage is exposure to extreme temperatures damaging the profile of the vaccine. This problem is particularly severe in underdeveloped and developing countries where continuous cold chain infrastructure doesn’t exist or cold-chain breaks are regular owing to intermittent electricity outages and poor handling. To counter this, several efforts are being made by global companies and research institutions on thermostable vaccines. Hilleman Labs in India, a joint effort of Merck and Wellcome Trust, is developing a heat-stable adaptation of Merck’s existing Rotavirus vaccine by using a stable, dry formulation with an optimized presentation format. Stabilitech, from UK, have utilized their protein free stabilization platform involving non-toxic and inexpensive chemical excipients mimicking natural phenomena to confer thermo-stability to live viruses, proteins including antibodies and alum adjuvant subunit vaccines. If commercialized, these thermostable vaccines, can not only reduce vaccine wastage and ease logistics costs, but can also expand global reach of vaccines by enabling vaccine delivery in remotest locations.
The vaccine industry and global research system has heralded in an era with significant impact on vaccine preventable deaths across diseases. While they continue to conquer persistent problems such as malaria and dengue and develop therapeutic vaccines, their efforts to improve current vaccines deserves great credit. It is encouraging to see the intradermal and intranasal flu vaccine on the market in US. In competitive markets, such products will provide developers product differentiation and we look forward to a more robust portfolio of such products as we progress towards the needle free vaccine. At the other end, to be widespread reality the public health focused vaccines will need support from several stakeholders including public health funders and procurement bodies and national governments. While eliminating the cold chain entirely may not be a near term reality, product specific small, simple and elegant innovations provide great optimism for the future of immunization programs.
Authors
 Grow Beyond
Grow Beyond 

