
Indian drug regulations: Simplification and enabling ease of doing business
India’s Union Health Ministry to Amend New Drugs and Clinical Trials Rules, 2019 for Streamlining Test Licence and BA/BE Study Applications
 The Indian pharma industry enjoys a dominant global position in manufacturing of quality assured generic and biosimilar drugs. The “pharmacy of the word” reputation has been earned on a foundation of scale and competitiveness building on progressively expanding technical prowess. However, as the industry looks to the next decade, it stares at a rapidly evolving external environment and confounding headwinds. As the next phase of growth is scripted, multiple vectors of enablement will be critical. Industry has ambitious and steadfast plans to step up innovation and investments. There is rapid focus on geographic and product diversification. This is combined with focus on supply chain resilience and significant investments in API reshoring. Finally for the next phase of possibilities to be actualised, regulatory enablement will be an important anchor.
The Indian pharma industry enjoys a dominant global position in manufacturing of quality assured generic and biosimilar drugs. The “pharmacy of the word” reputation has been earned on a foundation of scale and competitiveness building on progressively expanding technical prowess. However, as the industry looks to the next decade, it stares at a rapidly evolving external environment and confounding headwinds. As the next phase of growth is scripted, multiple vectors of enablement will be critical. Industry has ambitious and steadfast plans to step up innovation and investments. There is rapid focus on geographic and product diversification. This is combined with focus on supply chain resilience and significant investments in API reshoring. Finally for the next phase of possibilities to be actualised, regulatory enablement will be an important anchor.
It is this context that makes last week’s regulatory announcement from Government of India highly consequential.
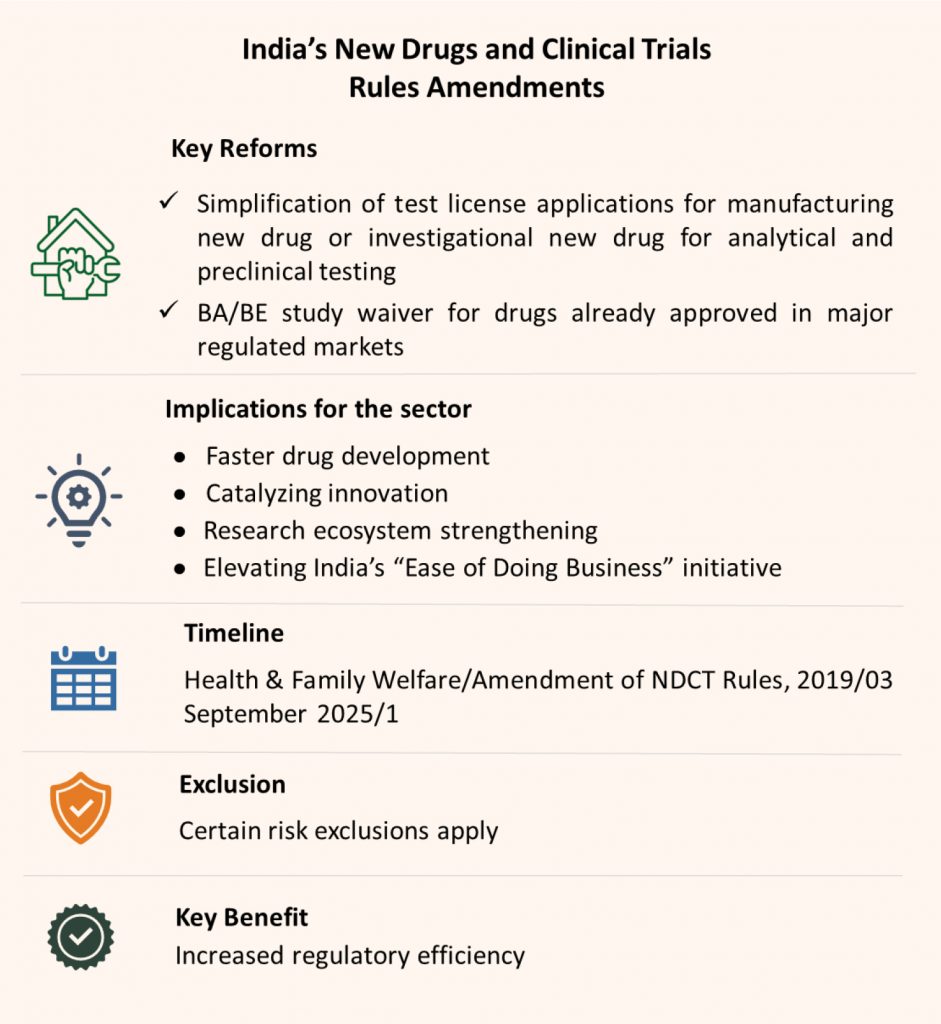
The Union Health Ministry’s proposed changes to the New Drugs and Clinical Trials (NDCT) Rules will significantly simplify and speed up test licensing and BA/BE study initiation, fostering an environment in India where drug development and clinical research can thrive. With the new draft amendments published for public comment in the Gazette of India on August 28, 2025, the government is strongly signalling its intent to make India an even more attractive hub for innovation, fast-track drug development, and global R&D collaborations.
What are the NDCT Rules, 2019 rules?
The NDCT Rules, 2019 were introduced by the Ministry of Health and Family Welfare, India, to establish clear and effective regulations for clinical trials and the approval of new drugs.
Application for permission to manufacture of new drug or investigational new drug (IND) for clinical trial or bioavailability and bioequivalence (BA/BE) study or for examination, test and analysis
- Any person/entity intending to manufacture a new drug or IND and conduct clinical trial or BA/BE study or for examination, or testing, shall submit an application in Form CT-10 to the Central Licencing Authority (CLA).
- No person/entity shall manufacture such drugs or conduct such studies without obtaining permission to manufacture from CLA.
Grant of permission to manufacture new drug or IND for clinical trial or bioavailability or bioequivalence study, or for examination, test and analysis
- CLA will review the Form CT-10 application, and if all requirements are met, it issues permission in Form CT-11 within 90 working days to manufacture the new drug/IND for clinical trial, BA/BE study, or testing.
- If the CLA does not respond within 90 working days, the permission to manufacture the new drug/IND for clinical trial, BA/BE study, or testing shall be deemed to have been granted and to be legally valid, allowing the applicant to proceed with manufacturing.
Validity period of permission to manufacture of new drug or investigational new drugs for clinical trial or bioavailability and bioequivalence study, or for examination, test and analysis
- Permission in Form CT-11 is valid for 3 years, unless suspended or cancelled by the CLA.
- In exceptional cases, the CLA may grant a 1-year extension if the applicant submits a written request with valid reasons.
Licence to manufacture new drugs or investigational new drugs for clinical trial or bioavailability or bioequivalence study or for examination, test and analysis under the Drugs and Cosmetics Rules, 1945
- After obtaining permission under rule 53, the manufacturer shall make an application for grant of licence to manufacture new drug or IND in accordance with the provisions of the Act and the Drugs and Cosmetics Rules, 1945.
Source: NDCT Rules, 2019, CDSCO
What’s changing
Simplification of Test License Applications
The amendment transitions the traditional test license requirement for manufacturing of new drug or Investigational new drug for analytical and preclinical testing to a notification/intimation-based process (except for select high-risk drugs).
- Applicants will no longer need to wait for explicit regulatory approval.
- Instead, they need to simply notify the Central Licensing Authority before proceeding, slashing the processing time from 90 to 45 days.
- This “notify and proceed” framework reduces delays and streamlines the pathway for drug testing and research.
In the New Drugs and Clinical Trials Rules, 2019, after the sub-rule (2) of rule 52, the following proviso shall be inserted, namely:
Provided that in case of manufacture of new drug or Investigational new drug for Analytical and preclinical testing (excluding the new drug and Investigational new drug of category of sex hormones, cytotoxic, beta lactam, Biologics with live microorganism and narcotics & psychotropic drugs) an online application shall be submitted as notification and applicant can manufacture such drugs based on the notification.
In the New Drugs and Clinical Trials Rules, 2019, in rule 53:
– Under the sub-rule (1), the words “ninety working days” shall be substituted with the words “forty-five working days” wherever occurs
– Under the clause (ii) of sub-rule (3), the words “ninety working days” shall be substituted with the words “forty-five working days”.
Source: CDSCO
BA/BE Study Applications
For certain categories of BA/BE studies—especially for drugs already approved in major regulated markets (US, EU, UK, Japan, Australia, Canada)—the existing requirement for a formal license to conduct BA/BE studies will be waived. These studies can commence upon submission of an online intimation to the regulator. This is a significant advantage for generic manufacturers and innovators working to demonstrate equivalence
- This shift will make it easier and faster to conduct BA/BE studies—a critical step in the approval of generics and new formulations.
- The reforms are expected to cut licence applications by nearly 50%, optimizing regulatory workflows.
Only drug classes deemed high risk (such as hormones, narcotics, cytotoxic agents, and live biologics) will continue to require the full licensing process.
In the New Drugs and Clinical Trials Rules, 2019, after sub-rule (2) of rule 31, the following proviso shall be inserted, namely:
‘Provided that in case of single-dose, two-period, two-sequence, two-treatment, bioavailability or bioequivalence studies in normal healthy adult human volunteers (for export purpose only), of oral dosage form of a drug (other than drugs of Cytotoxic, Hormone, Narcotic and Psychotropic substances categories and not a drug of Narrow Therapeutic Index or a drug having highly variable pharmacokinetics) already approved in the Country or any one of the countries namely, USA, EU, Japan, Australia, Canada and UK, the studies may be conducted after submission of an online application as notification and its acknowledgement by the CLA, subject to the following conditions:
– The application for the notification must be accompanied with approval of the ethics committee registered with CLA under rule 8
– The Ethics Committee shall maintain separately the record of review and approval of such BA/BE studies being conducted under this notification process which shall be reviewed by the CLA at the time of renewal of the Registration of the Ethics Committee”.
Source: CDSCO
Why this matters? What is the impact on India’s Pharma and Clinical Research
The proposed amendments to India’s New Drugs and Clinical Trials Rules, 2019 represent a pivotal shift in the country’s regulatory approach. This reform has far-reaching implications for India’s pharmaceutical and clinical research sectors, supporting faster drug development, catalyzing innovation, strengthening the research ecosystem, and elevating India’s “Ease of Doing Business” standing within global pharma.
The introduction of a “notify and proceed” framework eliminates unnecessary delays, reducing the processing time for approvals and allowing researchers to move forward more efficiently. This not only accelerates the initiation of clinical trials and BA/BE studies but also lessens the administrative burden on both regulators and applicants.
With licence applications expected to be cut by nearly 50%, regulatory workflows become more efficient. Drug classes considered high risk—such as hormones, narcotics, cytotoxic agents, and live biologics—will continue to require full licensing, ensuring safety is maintained where it is most critical.
Waiving formal licence requirements for BA/BE studies involving drugs already approved in major regulated markets allows generic manufacturers and innovators to pursue a path to market is more time efficient and is not burdened by regulatory redundancies.
◄ Key Implications ►
Accelerate drug development times – competitive advantage
Quicker initiation of clinical trials enables pharmaceuticals to bring new drugs to market much faster, reducing both time and costs.
Boost Innovation – Simplified procedures reduce license volumes, freeing up regulatory bandwidth which encourages Indian startups and academic institutions in developing new therapies.
Strengthens Research ecosystem – Contract Research Organizations (CROs) and Clinical Development & Manufacturing Organizations (CDMOs) thrive in environments with predictable, transparent, and swift regulations. This change enhances India’s positioning as a preferred global outsourcing destination. Global major pharma players looking to conduct multi-country clinical trials will view India as a strategic location to scale studies across diverse patient populations.
Broader vision – Ease of Doing Business in Pharma
The amendments are part of India’s broader Ease of Doing Business initiative and reflect a regulatory environment increasingly aligned with international standards. These changes help India align more closely with international standards. An efficient regulatory environment—especially in conducting BA/BE studies—is vital for securing product approvals both domestically and for export. India already offers a cost advantage for trials and research; simplification will attract more global companies to outsource such studies to India. Taken together, these reforms provide a solid foundation for India to leverage its strengths in biosimilars, generics, and advanced therapies, while attracting greater foreign investment and fostering technology transfer and collaborations in high-value research areas.
Once finalized, these changes will mark a major shift in how India approaches drug research regulation—removing bottlenecks and giving both domestic and international stakeholders a smoother pathway for innovation.
Authors


Connect with Authors at: E-mail healthcare@sathguru.com
 Grow Beyond
Grow Beyond 

